Approach
In order to conduct a comprehensive health economic analysis, the approach involved mapping the ‘AS IS’ scenario (standard therapy of care) and ‘TO BE’ scenario (intervention with antibody biomarker screening) for the given medical intervention. This mapping encompasses the entire care pathway, identifying the different steps involved and evaluating their costs and health effects. In order to obtain a comprehensive overview of the treatment trajectories, both the available literature as well as expert opinions were consulted.
Based on the identified patient pathways, an interactive Markov model was developed to assess the cost-effectiveness of the intervention in comparison to the standard of care. The construction of the model includes defining relevant "health states" and determining the transitions between them. Transition probabilities are assigned to represent the likelihood of patients moving from one health state to another during the care pathway. By additionally linking the corresponding cost and health effect to each health state, it is possible to compare both care pathways in terms of cost-effectiveness.
The results were presented in terms of expected direct medical costs, quality-adjusted life-years, and the incremental cost-effectiveness ratio. This ratio indicates the incremental cost required to gain an additional quality-adjusted life-year over a five-year period, considering the perspective of healthcare payers.
The guidelines of the Belgian Healthcare Knowledge Centre (KCE) were taken into account to ensure the methodological quality, transparency, and uniformity of the health economic analysis. The following table provides an overview of the basic elements of the economic evaluation that were applied in this health economic assessment in accordance with the KCE guidelines:
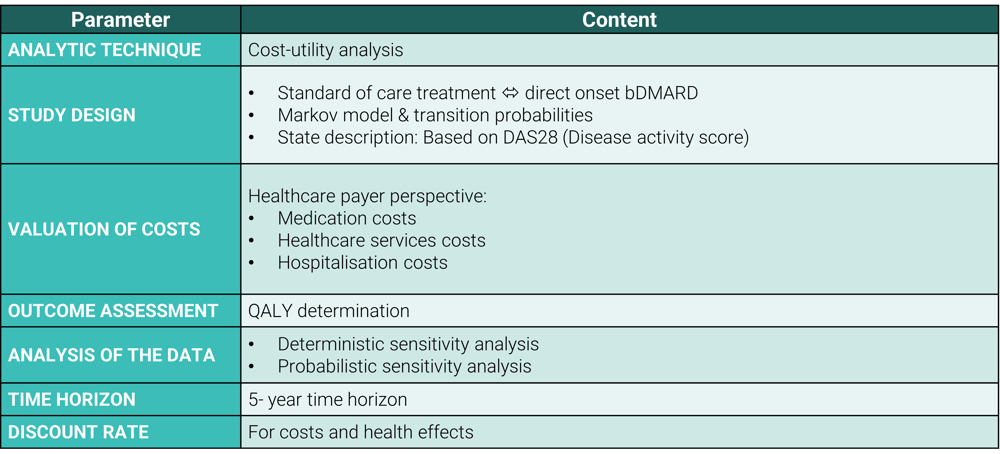
For the valuation of healthcare costs, the technical cell data from the FPS was used. To efficiently access this data, Möbius developed an interactive dashboard. Click here for more information on how to use this dashboard.
Results
A final report was drawn up - according to the reporting standards of the KCE - which focused on giving Uhasselt BIOMED a clear understanding of the methodology and initial results. This report includes a comprehensive explanation of the objectives, elements of economic evaluation, results, discussion, and references.
Additionally, a hands-on interactive model was delivered. Given the early stage in the research and limited clinical data, the interactive model can be used by the team at UHasselt BIOMED to adapt parameter values and test multiple scenarios in terms of cost-effectiveness.







